The High-Frequency Spectra of the Elements, I-II, in: The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, pp. 1024-34 in Sixth Series, Vol. 26, no. 156, December 1913 & pp. 703-13 in Vol. 27, no. 160, April 1914.
London: Taylor and Francis, 1913-14.
First edition, journal issues in original printed wrappers, and an exceptionally fine set of both parts of this landmark work, journal issue, in the original printed wrappers. “In 1913 and 1914, respectively, Moseley (1887-1915) published two papers which, once and for all, established a firm connection of the Periodic Table, which was based on empirical chemistry, to the physical structure of atoms” (Brandt, p. 97). “Moseley, working under Rutherford at Manchester, used the method of X-ray spectroscopy devised by the Braggs to calculate variations in the wavelength of the rays emitted by each element. These he was able to arrange in a series according to the nuclear charge of each element ... These figures Moseley called atomic numbers. He pointed out that they also represented a corresponding increase in extra-nuclear electrons and that it is the number and arrangement of these electrons rather than the atomic weight that determines the properties of an element. It was now possible to base the periodic table on a firm foundation, and to state with confidence that the number of elements up to uranium is limited to 92” (PMM). On the basis of his results, Moseley also predicted the existence of four new elements, later discovered and named hafnium, rhenium, technetium and promethium.
“Before 1913 the order of the elements in the periodic system was universally taken to be given by the atomic weight. Although this caused some anomalies, such as that related to the ‘reversed’ atomic weights of tellurium (Te = 127.6) and iodine (I = 126.9), the convention or dogma of atomic weight being the defining property of a chemical element was rarely questioned … According to Charles Galton Darwin, who at the time was a lecturer at Manchester University, the 1913 scattering experiments of Geiger and Marsden convinced Rutherford and his group that the nuclear charge was the defining quantity of a chemical element. The idea certainly was in the air, but it took until November 1913 before it was explicitly formulated, and then from the unlikely source of a Dutch amateur physicist. Trained as a lawyer, Antonius van den Broek had since 1907 published articles on radioactivity and the periodic system … In a short communication to Nature dated November 10 he disconnected the ordinal number from the atomic weight and instead identified it with the nuclear charge N (or Z, as it subsequently became symbolized). This hypothesis, he said, ‘holds good for Mendeleev’s table but the nuclear charge is not equal to half the atomic weight’. Van den Broek’s suggestion was quickly adopted by Soddy, Bohr, and Rutherford and his group … In an address of 1934 celebrating the centenary of Mendeleev’s birth, Rutherford credited Bohr for first having recognized the significance of an ordinal number for the chemical elements: ‘The idea that the nuclear charge of an element might be given by its ordinal or atomic number was first suggested and used by Bohr in developing his theory of spectra. By a strange oversight, Bohr himself gave the credit of this suggestion to van den Broek, who later discussed the applicability of this conception to the elements in general’ …
“Besides the successes from the spectra of hydrogen and helium, the strongest experimental support for Bohr’s theory came from X-ray spectroscopy, a branch of science that did not yet exist when Bohr completed his trilogy … The existence of monochromatic X-rays characteristic of the element emitting the rays had been known since 1906, when the phenomenon was discovered by Charles Glover Barkla, a physicist at the University of Liverpool. Although Barkla could not determine the wavelengths of the characteristic rays he could study and classify them by means of their penetrating power. He soon found that there were two kinds of rays, which he named K and L radiation and where the first had a greater penetrating power than the latter. What was missing, among other things, was a method of determining the wavelength of the radiation, but such a method was provided after William Henry Bragg and his son William Lawrence Bragg in 1912 invented the X-ray spectrometer based on the reflection of X-rays on crystals.
“In Manchester, Henry Gwyn Moseley, who was Bohr’s junior by two years, set out to employ the method of the Braggs to measure and understand the wavelengths of the characteristic radiation. He had earlier collaborated with Darwin on X-ray diffraction, but from the summer of 1913 he pursued the new research programme alone. Bohr knew Moseley, but it was only in July 1913 that he had a long discussion with him and told him about his new atomic theory. The two physicists evidently had shared interests, such as the periodic system and its relation to the atomic number. Moseley’s research programme was to a large extent motivated by the possibility of confirming by means of X-ray spectroscopy van den Broek’s hypothesis – or the van den Broek-Bohr hypothesis – of the atomic number. ‘My work was undertaken for the express purpose of testing Broek’s hypothesis, which Bohr has incorporated as a fundamental part of his theory of atomic structure’, he wrote. Moseley constructed a new kind of X-ray tube where the targets could be easily interchanged and moved in position opposite to the cathode, to give out their characteristic rays. To determine the wavelengths he developed a photographic method. Having surmounted the inevitable experimental difficulties, in October 1913 he was ready to collect data, starting with the K lines from calcium to zinc” (Kragh, pp. 32-3 & 104).
“In a very short time, Moseley produced the first of his two famous papers in which he showed the spectra of K radiation of ten different substances … Moseley arranged the spectra, one below the other in a step-like fashion, in such a way that a given wavelength was in the same position for all spectra. It then became clear by simple inspection of this ‘step ladder’ that the spectrum of K radiation of each element contains two strong lines (which Moseley called Kα (for the longer wavelength) and Kβ (for the shorter) and that this pair of lines moves to shorter and shorter wavelengths in a monotonic fashion if one moves step by step from calcium to zinc.
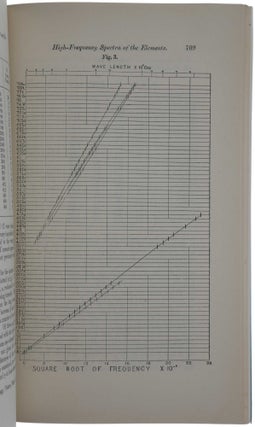
“Only a few months before Moseley’s work, Bohr had published his model of the atom with Z electrons, each of electric charge –e circling an atomic nucleus of charge Ze. Bohr had taken the nuclear charge number Z to be identical with the position number of the corresponding element in the Periodic Table. His theory could explain the visible spectra of the hydrogen atom (Z = 1) and the positive ion of helium (Z = 2) with only one electron. But he could not make calculations for atoms with more electrons. Moseley realized that, in contrast to visible spectra, the characteristic X-ray spectra, in particular the spectrum of Z radiation, was simple also for atoms of high Z. Since Bohr had conjectured that the electrons in an atom are arranged in separate rings and since in his model transitions to the innermost ring correspond to the highest energies, i.e., the shortest wavelengths, Moseley wrote: ‘The very close similarity between the X-ray spectra of the different elements shows that these radiations originate inside the atom, and have no direct connexion with the complicated light-spectra and chemical properties governed by the structure of its surface.’ Moseley also gave a formula describing the frequency of the K radiation for all elements which he had studied and predicting it for all others and, on the basis of very sparse data, even gave a similar formula for the L radiation. The formulae later were called Moseley’s law.
“Moseley's work made it clear once and for all that indeed the position number in the Periodic Table is equal to the number Z of positive elementary charges in the nucleus of an atom. It also showed that Z is more important for the spectroscopic and chemical properties of an atom than the atomic mass number A. This is evident in the case of the elements cobalt (Z = 27, A = 58.9) and nickel (Z = 28, A = 58.7), where even the order in A differs from that in Z.
“At this stage of his work Moseley decided to leave Manchester and to move back to Oxford, although Rutherford had offered him a fellowship for the academic year 1913/14, and although he got no paid position in Oxford. His motives are not entirely clear but it seems he thought that it would be easier eventually to obtain a professorship in Oxford if he was on the spot. With a grant of 1000 Belgian Francs from the Solvay Foundation he set up new equipment in Townsend’s laboratory, where he was allowed to work as a guest … With Moseley’s technique and Moseley’s law it was easy to determine the number Z for virtually any known element. For elements with higher values of Z the L radiation had to be used, since the voltage available for X-ray tubes was not high enough to produce the K radiation with its shorter wavelength. Already in April 1914 Moseley published his results [the second offered paper]; one comprehensive diagram contains the frequencies of K or L lines for most elements between aluminium (Z = 13) and gold (Z = 79). In the conclusions he wrote: ‘Known elements correspond with all numbers between 13 and 79 except three. There are here three possible elements still undiscovered.’ These were the elements with Z =43, 61, and 75. In fact, also the element with Z = 72, taken to be a rare earth, was missing. Moseley had assumed its existence, because it was reported in the chemical literature, but could not get a sample of it to use in his measurements. All four elements were found between 1922 and 1945, two in terrestrial material (hafnium, Z = 72, and rhenium, Z = 75). The other two had to be produced by nuclear reactions (technetium, Z = 43, and promethium, Z = 61) since these radioactive elements do not seem to exist in the earth’s crust.
“Moseley’s family background and education were exceptional. His father, Henry Nottidge Moseley, and both his grandfathers, Henry Moseley and John Gwynn Jeffreys, were Fellows of the Royal Society. His father, who had been professor of zoology at Oxford, died when Moseley was only four years old. From then on his mother saw to it that he got the best education available. In 1901 he won a King’s scholarship for the prestigious Public School of Eton and in 1906, again with a scholarship, he entered Trinity College, Oxford. He studied physics under Townsend and, after graduating in 1910, joined Rutherford’s outstandingly successful group in Manchester. He did some work on radioactivity but, immediately after learning of Laue’s theory of X-ray diffraction and the experiment by Friedrich and Knipping in the summer of 1912, he became focused on X rays.
“Moseley was invited to report on his work at the meeting of the British Association for the Advancement of Science held in Australia in August 1914. It was the month in which the First World War began. Immediately after his talk Moseley travelled back to England by the next steamer to volunteer for the army. He even ‘pulled private strings’ and became a lieutenant in the Royal Engineers. On 10 August 1915, he perished in the Battle of Sari Bair” (Brandt, pp. 97-101).
Norman 1599; Printing and the Mind of Man 407. Brandt, The Harvest of a Century, 2009. Kragh, Niels Bohr and the Quantum Atom, 2012.
Two complete journal issues, 8vo, pp. ???. Original printed wrappers, untouched. Very rare in such fine condition.
Item #4607
Price: $9,500.00