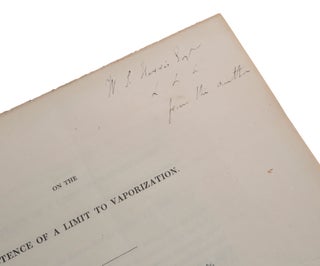On the existence of a limit to vaporization. Offprint from: Philosophical Transactions, Vol. 116. Read before the Royal Society, June 15, 1826.
London: W. Nicol, 1826. First edition, very rare separately-paginated offprint, inscribed by Faraday to his friend and fellow electrical researcher William Snow Harris. This early chemical paper of Faraday contends that, while every substance is normally surrounded by a vapour of the same substance, and that the pressure of this vapour increases with temperature, when the temperature is lowered sufficiently the vapour pressure vanishes, so that the body becomes ‘fixed.’ “What puts a stop to vaporization? it may be asked. Liquids, we know, have a certain attraction for their own particles, evinced in their disposition to collect into drops. The particles of solids are attracted more powerfully, and cohere strongly together. Mr. Faraday is of opinion, that when the vaporizing power becomes weak, at low temperatures, it may be overcome and negatived completely by this cohesive attraction, and no escape of particles in the vaporous form be permitted” (Graham, p. 76). Modern physical chemists would dispute this conclusion, but Faraday’s paper is nevertheless important for what it tells us about Faraday’s evolving ideas on the nature of matter, and specifically his growing acceptance of Boscovich’s atomic theory, which he had published in his great work Theoria naturalis philosophiae (1758). According to this theory, atoms are points surrounded by continuously varying forces that are alternately attractive and repulsive according to the distance from the point, and so can be represented by a curve which has a series of maxima and minima. “Although he did not publicly announce his commitment to the theory of point atoms until 1844, Faraday worked within this framework from his earliest productive years. The fact that Davy and Faraday knew that their atomic theories would be dismissed as metaphysics at best or meaningless at worst led them both to keep them in the background … It was not until the 1820s when [Faraday] began to wrestle more closely with theoretical questions that the hypothesis of point atoms finally was accepted” (Pearce Williams, pp. 77-8 & 89). Faraday’s paper was published in the Phil. Trans., vol. 116, pp. 484-93. This separately-paginated offprint precedes the journal publication, as indicated by the printed statement on the verso of the title page: “Gentlemen who are indulged with separate Copies of their Communication, are requested to use their endeavour to prevent them from being reprinted, till one month after the publication of that part of the Philosophical Transactions in which they were inserted.” OCLC lists copies of this offprint in the US at Columbia and Huntington (ex Burndy) only. No other copies in auction records. Provenance: “Faraday’s friend W[illiam] Snow Harris” (Pearce Williams, p. 303) – inscribed by Faraday ‘W. S. Harris Esq. from the author’ on title. Harris was a British physician and electrical researcher, nicknamed ‘Thunder-and-Lightning Harris,’ and noted for his invention in 1820 of a successful system of lightning conductors for ships. One of the successful test vessels was HMS Beagle which survived lightning strikes unharmed on her famous voyage with Charles Darwin. Harris (1791-1867) was born in Plymouth, studied medicine at the University of Edinburgh, then returned to Plymouth and set up a medical practice. In 1824 he married, and decided to abandon medicine to concentrate on his studies of electricity. His paper ‘On the Relative Powers of various Metallic Substances as Conductors of Electricity’, read before the Royal Society in 1826, led to him being elected a fellow of the society in 1831. The decade of the 1820s was the period of Faraday’s most intensive activity in the area of chemistry. “Two problems particularly engaged his attention: one was the seemingly anomalous behaviour of chlorine and the other was the more general problem of molecular forces and the states of matter … “In 1820, writing to Professor G. de la Rive at Geneva, …Faraday mentioned a fact which he found surprising. ‘The positive effect,’ he told de la Rive, ‘is the volatilization of silver. We often have it in our experiments sublimed into the upper part of the crucible and forming a fine dew on the sides and cover so that I have no doubt at present on the volatility of silver though I had before.’ If silver were volatile, how many other ‘solid’ substances also had atmospheres of their own vapours surrounding them and to what distance did this atmosphere extend? These were questions that could be answered by experiment and the answers could then be applied to test a hypothesis of the nature of the physical states of matter which Faraday had first encountered in 1812. At the first lecture he attended at the Royal Institution, he heard Davy state: ‘In solids the matter exerts its attractive powers and remains fixed. In fluids there is an equilibrium between the attractive and repulsive powers. In Gasses the repulsive power preponderates over the Attractive but is not sufficiently strong to overcome the specific nature of the body and in Radiant matter the repulsion is free and unconfined and projects the body in right lines.’ “The physical states of matter, then, were merely functions of the two powers of attraction and repulsion with which ‘matter’ was associated. By ‘exalting’ those powers through the agencies of heat and electricity, their effects under different conditions could be studied. If it were shown, as Faraday now suspected, that every body could be made to exist in a solid, liquid, and gaseous form, it would provide unassailable experimental proof for the philosophical principle of continuity upon which both Kant and Boscovich had so insisted … “To Faraday, to conceive an idea was tantamount to conceiving an experiment to test it. His first essay was a simple one: he suspended a piece of gold over some mercury in a bottle and put the whole thing away in a dark place for a while to see what would happen. The results were encouraging: the gold was whitened by the mercury vapour that condensed upon it, and Faraday was able to report, ‘at common temperatures, and even when the air is present, mercury is always surrounded by an atmosphere of the same substance.’ From the example of the silver and the mercury, it was possible to conclude that all bodies, under suitable circumstances, could pass into the state of vapour. The next question to be resolved was the distance to which this atmosphere of vapour extended. The problem was of considerable theoretical importance for the existence of such vapours under all conditions had long been assumed, and this assumption could not be experimentally justified. ‘It is well known [he wrote] that within the limits recognised by experiment, the constitution of vapour in contact with the body from which it rises, is such, that its tension increases with increased temperature, and diminishes with diminished temperature; and, though in the latter case we can, with many substances, so far attenuate the vapour as soon to make its presence inappreciable to our tests, yet an opinion is very prevalent, and I believe general, that still small portions are produced; the tension being correspondent to the comparatively low temperature of the substance. Upon this view, it has been supposed that every substance in vacuo or surrounded by vapour or gas, having no chemical action upon it, has an atmosphere of its own around it; and that our atmosphere must contain, diffused through it, minute portions of the vapours of all those substances with which it is in contact, even down to the earths and metals. I believe that a theory of meteorites has been formed upon this opinion’ [p. 3]. “One might well ask here if this was not precisely Faraday’s position? Had he not just finished establishing that every body is surrounded by a gaseous atmosphere of its own molecules? And would not the proof of a limit to this process of vaporization destroy the very principle of continuity which Faraday was supposedly so eager to uphold? The apparent contradiction vanishes if we appeal again to the theory of point atoms and recall Faraday’s contention that all bodies will vaporize under certain conditions. In the theory of point atoms it was possible to have the discontinuity implied by suggesting a limit to vaporization without sacrificing the principle of continuity itself. If the gaseous state were defined in terms of intermolecular distance, then reference to the Boscovichean curve will immediately reveal a limit to vaporization although the curve itself is continuous. Until two particles can leap over the hump of repulsion and pass from one stable point to another, change of state will not take place. The volatilization of silver would occur when, by heat, the repulsive power of the silver molecules was ‘exalted’ to permit the required separation. Upon cooling, as the repulsive force lost its ‘exaltation’, a critical point would be reached when the attractive and repulsive forces just balanced. Any further diminution of repulsion would push the particle into an attractive arch and the intermolecular distance would become that of solid silver. Faraday did not present the argument in these terms. Rather, he wrote: ‘The metal, silver, for instance, when violently heated, as on charcoal urged by a jet of oxygen, or by the oxy-hydrogen, or oxy-alcohol flame, is converted into vapour; lower the temperature, and before the metal falls beneath a white heat, the tension of the vapour is so far diminished, that its existence becomes inappreciable by the most delicate tests. Suppose, however, that portions are formed, and that vapour of a certain tension is produced at that temperature; it must be astonishingly diminished by the time the metal has sunk to a mere red heat; and we can hardly conceive it possible, I think, that the silver should have descended to common temperatures, before its accompanying vapour will, by its gradual diminution in tension, if uninfluenced by other circumstances, have had an elastic force far inferior to the force of gravity; in which case, that moment at which the two forces had become equal, would be the last moment in which vapour could exist around it; the metal at every lower temperature being perfectly fixed’ [pp. 6-7]. “Again, it is impossible to prove that Faraday was thinking in terms of point atoms. But the passage can be interpreted intelligently in these terms. That Faraday, by the time that this article was written (1826), was more or less committed to this theory will be shown when his work on the liquefaction of gases is considered. All Faraday did here was to end on a tantalizing note. ‘I refrain from extending these views, as might easily be done, to the atomic theory, being rather desirous that they should first obtain the sanction or correction of scientific men’ [p. 11]. Would an extension of these views to the atomic theory have introduced atomic ‘limits’ of powers? Would these not, then, have had to follow the essential shape of Boscovich’s curve?” (Pearce Williams, pp. 125-7). Graham, Elements of Inorganic Chemistry, 1858. Pearce Williams, Michael Faraday, 1965.
Large 4to (292 x 232 mm), pp. [1-3], 4-12 (single vertical crease for posting, other light creases). Disbound (paper spine strip). Very good.
Item #5299
Price: $1,850.00