A New System of Chemical Philosophy.
Manchester: S. Russell for R. Bickerstaff; Russell & Allen for R. Bickerstaff; Executives of S. Russell for G. Wilson, 1808; 1810; 1827. First edition, untouched in uniform original publisher’s bindings with original printed spine labels, of Dalton’s classic work on the atomic theory of matter. “Dalton reconstructed Newton's speculations on the structure of matter, and, applying them in a new form to chemistry, gave Lavoisier's reformation of that science a deeper significance” (PMM). “Dalton's chemical atomic theory was the first to give significance to the relative weights of the ultimate particles of all known compounds, and to provide a quantitative explanation of the phenomena of chemical reaction. Dalton believed that all matter was composed of indestructible and indivisible atoms of various weights, each weight corresponding to
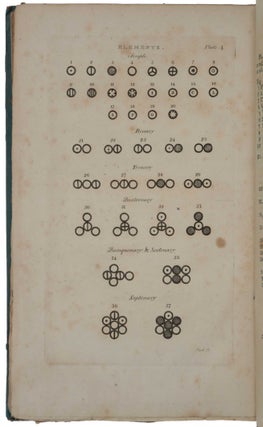
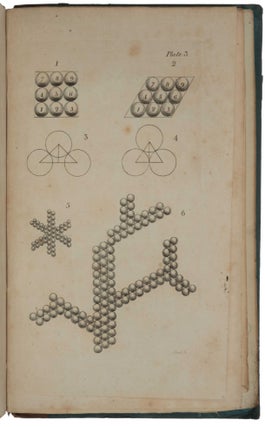
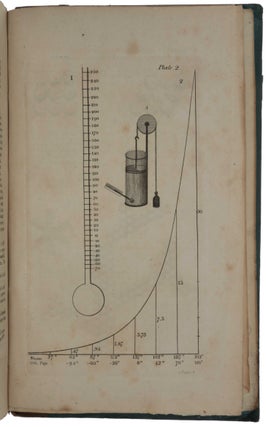
one of the chemical elements, and that these atoms remained unchanged during chemical processes. Dalton's work with relative atomic weights prompted him to construct the first periodic table of the elements (in Vol. I, pt. 1), to formulate laws concerning their combination and to provide schematic representations of various possible combinations of atoms. His equation of the concepts "atom" and "chemical element" was of fundamental importance, as it provided the chemist with a new and enormously fruitful model of reality” (Norman). “He developed a system of chemical symbols and a table [plate 4 in part 1] showing the relative weights of the atoms of a list of elements. From his principles he deduced the law of definite proportions and the law of multiple proportions” (Dibner). This, and pp. 546-548 along with the 4 plates in part 2, in effect describe the first periodic table, which Mendeleev was to refine later. “In 1808 John Dalton published A New System of Chemical Philosophy, which described principles such as the uniqueness of atoms of the same element, relative atomic masses, and the rules of chemical combination, which taken together comprise the tablets of modern chemistry. The adoption of molecular formulas based on the laws of definite and multiple proportions, and the assignment of relative atomic masses, placed the science on a new quantitative footing” (Grossman, p. 339). This set is complete with the two parts of Vol. I and Vol. II, Part I (no further parts were published). Only one complete copy in uniform publisher’s bindings has appeared since Norman, but that copy was made up with varying provenances, one part being ex-library. The Norman copy itself was in non-uniform bindings without the printed paper spine labels. Dalton (1766-1844) was born of a Quaker family at Eaglesfield, a small village in the English Lake District. As a young boy, he worked in the fields with his older brother, and helped his father in the shop where they wove cloth. In 1776, when only ten years old, he entered the service of Elihu Robinson, a wealthy Quaker, who taught him mathematics. In 1781, after a brief period of teaching in the village school, he joined his brother who was a master at a school in Kendal. In 1793 he moved to Manchester and, in the same year, published Meteorological Observations in which he suggested that the processes of evaporation and condensation were not chemical changes of state but rather changes in the physical form of water. These ideas laid the foundations for his theory that all matter is composed of discrete ‘atoms’. At first after he moved to Manchester he taught mathematics and natural philosophy at New College, and began observing the behavior of gases, but after six years he resigned. Thereafter he devoted his life to research, which he financed by giving private tuition. By 1800, Dalton had become the secretary of the Manchester Literary and Philosophical Society (LPS), and in 1805 he presented a series of papers to the society outlining key points about the behaviour of gases in his series of essays, “Experimental Essays on the Constitution of Mixed Gases”. He proposed that particles of an elastic fluid or gas were in fact elastic only with particles of their own kind. This extended Boyle’s law to mixtures of gases. Dalton’s further essay “On the Expansion of Gases by Heat” proposed the law that all elastic fluids expand the same quantity at the same heat. Jacques Charles (1746-1823) and Joseph Louis de Guy-Lussac (1778-1850) arrived at the same notion almost simultaneously. It is in this work that we see interest in the particles of matter themselves, rather than their behaviour, begin to emerge in Dalton’s thinking. Atomism itself was not a particularly new idea in the early 19th century. The ancient Greek philosopher, Democritus (460–370 B.C.E.), proposed as part of a materialist philosophy that matter was made of tiny, indivisible pieces. Democritus suggested that there were certain basic elements that could be neither created nor destroyed, but that could be arranged into myriad forms. These atoms possess simple properties only, specifically size, shape, and mass. Anything else that we perceive, such as colour, is merely the result of interaction between the particles. In the 17th century, Isaac Newton hypothesized that matter was made up of atoms—small, hard homogeneous spheres adhering to one another through the gravitational force they exerted. The notion that matter was composed of atoms, and that there existed pure substances made of only one kind of atom (elements), was fairly common during the later Enlightenment period and into the 19th century. As a member of the Manchester LPS, Dalton was certainly familiar with these ideas. Dalton came to the notion of atomic structure only gradually, at first as a physical concept forced on him by his study of the atmosphere, heat, and gases. Dalton’s notebooks and papers were destroyed during the bombing of Manchester during World War II, so historians of science perforce rely on earlier generations for their reporting of Dalton’s day-to-day investigations. In his 1802–1804 notebooks, Dalton records on September 6, 1803, a table of the relative weights of the atoms of several substances, including water, carbon dioxide, and ammonia. With his assumption that matter always combines in the simplest possible manner, he used this chemical analysis to arrive at the idea that the gases he observed were made of atoms of differing weights, of different elements. Extending this idea to matter more generally, Dalton formulated his theory of atomism, which contains four basic parts. The first part is that chemical elements are made of atoms. These atoms are minute, discrete, indestructible particles invisible to the technology of the day. Dalton’s idea that an element is a chemical substance that cannot be further reduced to atoms derives from Antoine-Laurent de Lavoisier (1743-94). While Dalton’s atoms are frequently imagined as featureless balls, Dalton never ruled out the possibility of subatomic structure. The second and third parts of Dalton’s theory of chemical atomism holds that the atoms of an element are identical to each other and different from those of other elements. This was Dalton’s law of constant composition. Although chemists had long claimed that different elements had different weights, no one had been able to work out the values of those weights. Dalton was the first to do so. The fourth part of his theory held that elements combine in small whole-number ratios. The relative number of atoms in a given compound is consistent from sample to sample. This explained Joseph Louis Proust’s (1754-1826) law of definite proportions (1797). Water, for example, will always consist of two parts hydrogen and one part oxygen, a simple ratio of 2:1. This idea likewise explained Dalton’s own law of multiple proportions, derived from his observation that some atoms occur in different proportions with the same elements. For example, carbon and oxygen can produce carbon monoxide and carbon dioxide. The masses of one element combine with a fixed mass of a second element. Thus, the amount of carbon changes but always in relation to the same mass of oxygen. In 1808 Dalton began the publication of his great work, A New System of Chemical Philosophy, in which he developed the ideas he had been working on since 1801 concerning heat and chemical combination and laid the foundation for systematic chemical notation by graphically representing the arrangements of atoms in compounds. Dalton explains the publication strategy of his New System in his Preface: he first intended ‘to publish it intire in one volume’, but changed his mind in order to ‘exhibit and elucidate ... those primary Laws, which seem to obtain in regard to heat, and to chemical combinations’ as swiftly as possible, being warned by colleagues that ‘the interests of science, and his own reputation might suffer by delay’. Since his exposition of ‘the doctrine of heat, and the general principles of Chemical Synthesis, are in a good degree independent of the future details, there can no detriment arise to the author, or inconvenience to his readers, in submitting what is already prepared, to the inspection of the public.’ Hence Dalton put into print the essential ‘Part I’ of his New System in May 1808, reserving the ‘details’ of his experiments and analysis for two years: that supplement, entitled ‘Part II’, appeared in 1810, with a prefatory apology for its two-and-a-half year delay, and with its pagination continued from that of Part I. A very belated third part (described as ‘Volume II, Part I’, but effectively a new work under the old title) saw print only in 1827. “By the time this part appeared much of it was out of date” (Partington, p. 799). “It is traditional to locate Dalton’s New System of Chemical Philosophy as a step — perhaps the greatest — in a long road to modern atomic theory that began with the ancient Greek atomists Leucippus and Democritus in the fifth century BC, and ended with the nuclear atoms proposed by Ernest Rutherford and Niels Bohr in the early twentieth century, then quantum theory and scanning probe microscopes. The ‘philosophy’ in Dalton’s title signified something closer to a scientific theory than to the abstract reasoning it tends to connote today. Yet his book also represents an important juncture for the philosophy of science. It spoke to whether science should be based on empiricism or explanatory hypothesis — a question that had exercised Newton and Robert Boyle in the seventeenth century” (Ball). Dalton’s theory was not universally or immediately accepted. One critic wrote, “Atoms are round bits of wood invented by Mr. Dalton.” More damagingly, Sir Humphrey Davy (1778-1829), easily the most famous English chemist of the age, described Dalton as a rough experimenter who could usually be counted on to find the results he sought, relying on his head rather than empiricism. Dalton wrote in the preface to the second part of the first volume of his New System that he had so often been misled by the results of others that he was determined to trust only what he verified. To others, however, this too often looked like a lack of receptivity to other, perhaps contradictory, ideas. Despite such charges, Dalton gradually gained recognition and even honour for his contribution. He was elected a Fellow of the Royal Society in 1822, and eventually a foreign associate of the Paris Academy of Sciences. In 1833, the British government granted him a pension of £150, which was doubled in 1836. In the mid-19th century, this was a lavish sum. But perhaps the greatest honour is this: The units of atomic mass, while now called unified atomic mass units (amus), were once called “daltons”. Dibner, Heralds of Science 44; Duveen p. 156; Horblit 22; Norman 575; Partington III, pp. 799-813; Printing and the Mind of Man 261. Ball, ‘A New System of Chemical Philosophy,’ Nature, vol. 537 (2016), pp. 32-33. Grossman, ‘John Dalton and the London atomists,’ Notes and Records of the Royal Society of London, vol. 68 (2014), pp. 339-356. Partington, AHistory of Chemistry, Vol. 3, 1962 (see Chap. XVII). Smyth, John Dalton 1766–1844. A Bibliography of Works by and about Him, 9.
Two vols. (comprising three parts) bound in three, 8vo (225 x 137 mm), pp. vi, [ii], 220, with 4 plates; [viii], 221-560, with 4 plates; xii, 357, [3]. Original publisher’s calf-backed boards with printed paper spine labels (spines worn with some loss, front board of second volume loose. Preserved in a box. A completely genuine, untouched copy, in uniform publisher’s bindings.
Item #5935
Price: $65,000.00